<A>
electrons: *

On the left is an oxygen atom with 8 electrons orbiting at different levels. The nucleus contains 8 protons (positive charge) & 8 neutrons (no charge).

This diagram schematically shows 3 energy levels. Orbitals are grouped in zones at different distances from the atomic centre or nucleus. Electrons in zones close to the centre are lower in energy than electrons in zones at greater distances from the centre.
According to Bohr, the amount of energy needed to move an electron from one zone to another is a fixed, finite amount (a quantum). These zones are known as energy levels or sometimes electron shells.

<B>
thermodynamics (quanta): *
The law of conservation of energy states that the total energy of an isolated system is constant; energy can be transformed from one form to another, but can be neither created nor destroyed. The first law of thermodynamics is a version of the law of conservation of energy adapted for thermodynamic processes; it identifies 2 kinds of transfer of energy, heat and thermodynamic work, and relates them to a function of a body's state, called Internal energy. The formula for the First LAw of thermodynamics.

<C>
Rutherford (his atom): *
His most famous work was done in Manchester with H. Geiger and E. Marsden in the gold foil experiment (1909). They demonstrated the nuclear nature of atoms by deflecting alpha particles passing through a thin gold foil. He instructed his team to seek for alpha particles with very high deflection angles (not predicted). These were found & Rutherford constructed his model of the atom: a body with a very small charged nucleus, containing much of the atom's mass, was orbited by low-mass electrons (1911). Though he could not prove it he theorized atoms have their charge concentrated in a very small nucleus.
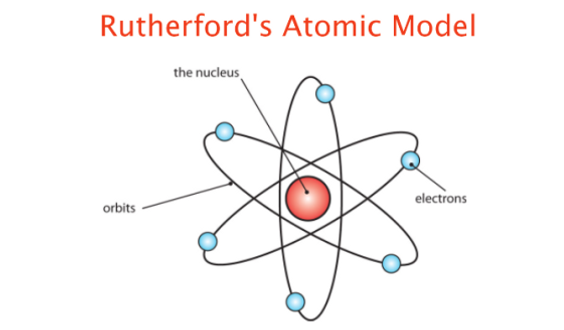
Rutherford envisioned the atom as a miniature solar system, with electrons orbiting around a massive nucleus, and as mostly empty space, with the nucleus occupying only a very small part of the atom. The neutron had not been discovered when Rutherford proposed his model, which had a nucleus consisting only of positively charged protons.
<D>
Niels Bohr (& the quanta" of Planck): *
In 1913 Niels Bohr adapted Rutherford's nuclear structure to Max Planck's quantum theory & created his Bohr model of the atom
The image below shows 3 levels of energy (n1, n2, and n3); an electron losses energy when it collapses into a lower orbit, from level 3 to level 2.


Electrons losing energy emit photons; in this case the electron losses energy moving from level 2 to level 1 and emits 1 photon
<E>
Planck (quanta): *
In 1894, Planck turned his attention to the problem of black-body radiation. The problem was how does the intensity of the electromagnetic radiation emitted by a black body (a perfect absorber) depend on the frequency of the radiation (i.e., the colour of the light) and the temperature of the body? Physicist had been working on this for nearly half a century without luck
see image

In 1900 Planck put forward his postulate to the German Physical Society: His formula successfully predicated predicted the curve of the black body radiation.
His formula was:
E=hv
E= energy, h= Planck’s constant (6.63 x 10-34), v= frequency.
Planck solved the black body problem theoretically by determining his Plank constant. This he derived from the emission & absorption of light, by examining the spectrum of a black-body radiator or the kinetic energy of photoelectrons. His formula accurately predicted the curve of the black body radiation.(see graph). His approach used the idea of a quantum. This is a discrete amount of energy. His postulate stated electromagnetic energy could be emitted only in quantized form, in other words, the energy could only be a multiple of an elementary unit. EMR is always emitted in quanta, specific packs of energy. This is his quantum theory. It was radical because it broke with Classical physics which was based on wave principles, not particles. Planck himself did not accept it, but it was the first milestone in Quantum Physics. Bohr would use the idea of quantum in his atom, in which the energy levels of electrons are discrete, the electrons revolve in stable orbits around the atomic nucleus but can jump from one energy level (or orbit) to another.
<F>
Leucippus and Democritus (atoms): *
Leucippus & Democritus posited that, since it was impossible to keep dividing matter infinitely, it must be made of extremely tiny particles, which were indivisible & could not be broken down: atoms. The solidness of the material corresponded to the shape of the atom: iron atoms are solid & strong with hooks that lock them into a strong solid; water atoms were smooth & slippery; salt atoms were sharp & pointed (owing to their taste), air atoms were light & whirling, pervading all other materials. Atoms differed from each other by their shape, size, & arrangement of their parts. Their connections were made of links such as hooks & eyes, or balls and sockets. This Democritean atom is an inert solid (merely excluding other bodies from its volume) & interacts with other atoms mechanically.
see image below illustrating water, salt & iron atoms
